You can author a document within eTMF. When documents are authored in eTMF, the user has the ability to check out the content while in Draft status, make changes, and upload the changes to the system. The user also has the ability to share authoring responsibilities with another user.
To finalize an authored document, it must be sent through an approval workflow (which may or may not be an eSignature workflow).
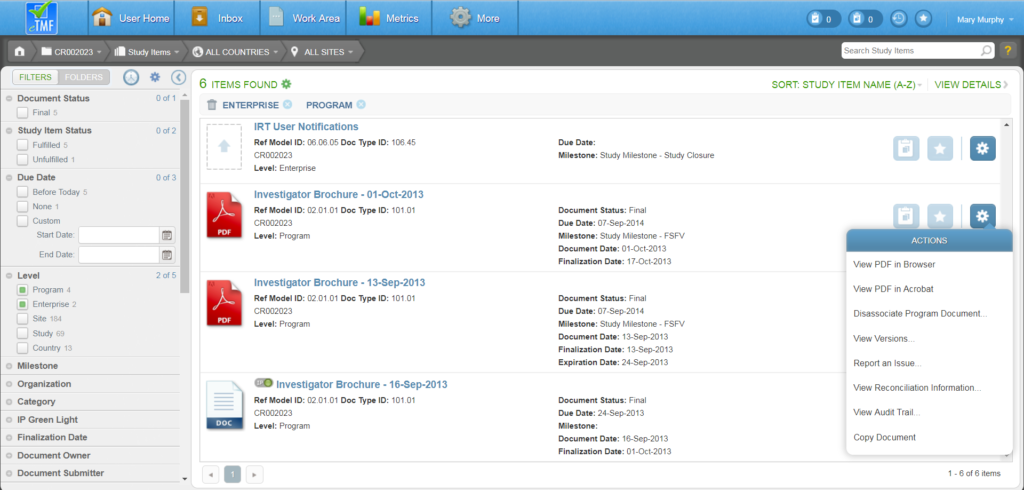
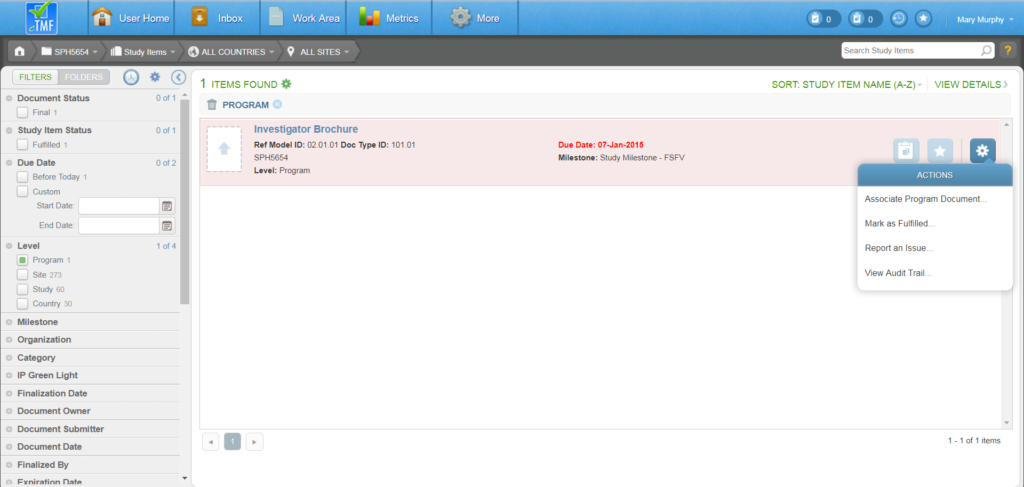
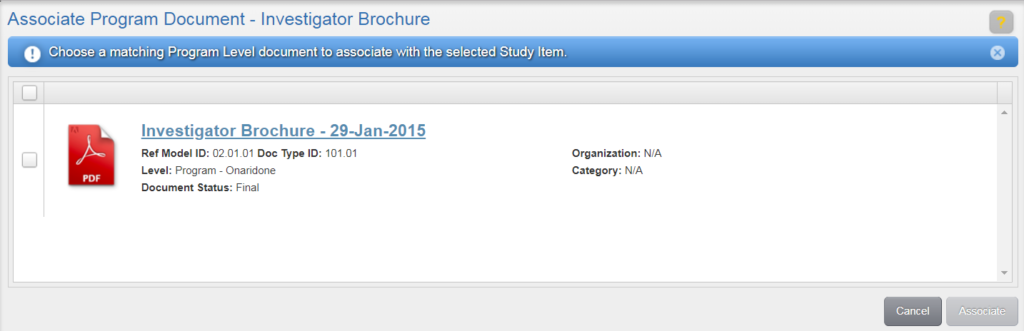
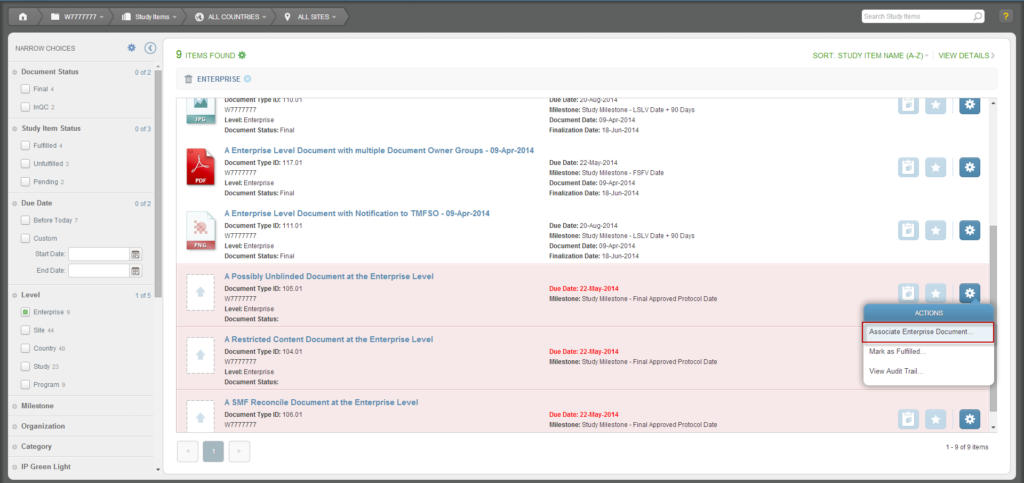
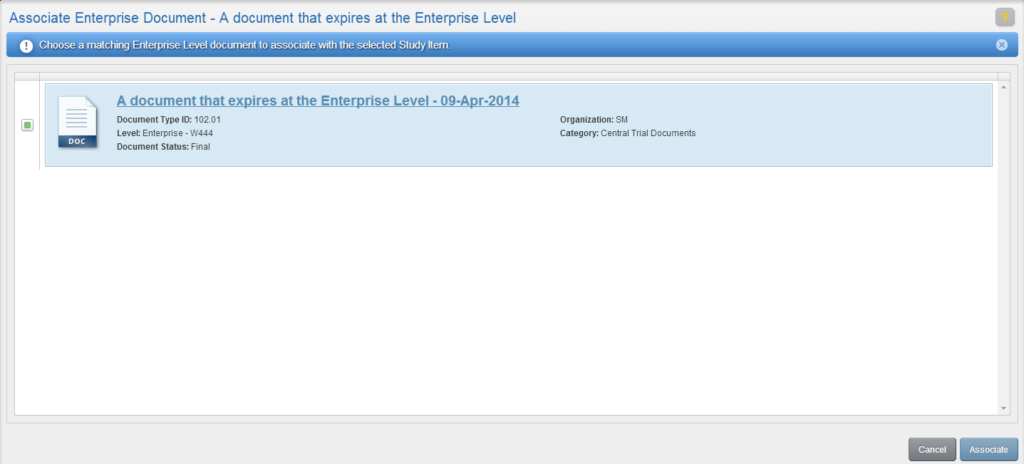
To author a document, navigate to your Work Area and click the Author Document button (the rightmost button). Or, from the Actions menu of an unfulfilled Study Item, select “Author New Content.”
Differences in the Two Ways to Author a New Document
Author Document Button (Work Area)
- Used if study item does not already existing on the study item list
- Must select the study to which the content belongs and, if you need to propose a new study item, select a document type from the master list
- Able to create content for enterprise or program level documents
- Able to select from list of applicable templates or import content
Author New Content (Unfulfilled Study Item)
- Used if study Item already exists on the Study Items List
- Allows for minimal Indexing
- Not available for Enterprise or Program level documents
- Able to select from list of applicable templates or import content
Authoring a New Document From a Template or by Importing Content
Using either of the methods above, you must choose the base content file. This file can be chosen from a list of existing document templates in the system or by importing an existing file.
Use a Document Template
A document template is typically used when the content does not yet exist. The template serves as the starting point to create the content of the document.
Note: Document templates are configured by Business Administrators and can be of any eTMF acceptable file type.
When you choose to author content, the list of document templates you can select from is limited to those applicable for the document type of the study item. You can limit the list by a specific language using the Template Language drop-down. If limiting by language, you can also select the adjacent check box to include English language templates in addition to those for the selected language.
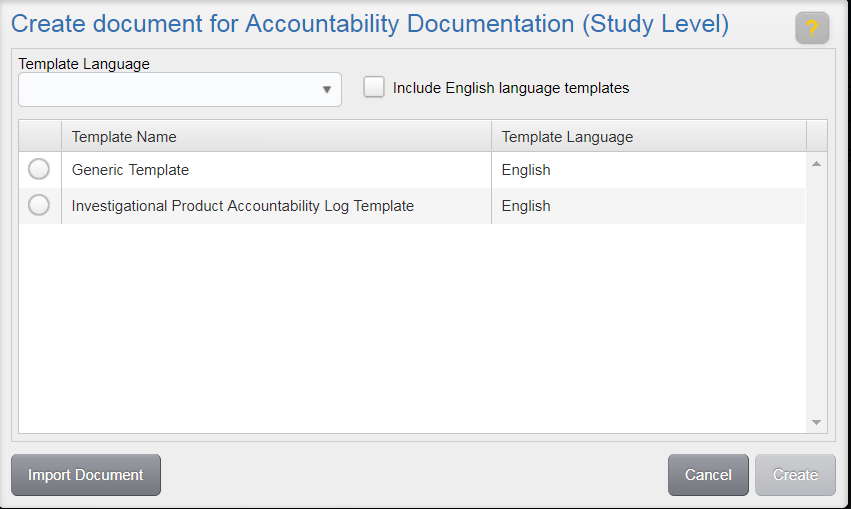
Once a template has been selected, click Create. This will add the document to your Work Area where you can continue authoring it by checking it out to your local machine.
Import a File
To import a document that has been partially authored outside the eTMF, click Import Document. This will display the Upload to Study Item screen. Click Select File, navigate to and double-click on the file you want to upload, and click Upload. The will add the document to your Work Area where you can continue authoring it by checking it out to your local machine.