Use a Program Level Document or Enterprise Level Document when studies need to reference documents not created specifically for that study. Program documents are documents associated with multiple clinical studies for a given program. For example, the Investigator Brochure generally applies to all ongoing studies for a program, as do certain safety documents. Enterprise documents are documents not associated with any clinical program. For example, validation documents are needed for systems such as IVRS and EDC applications, but these systems may be used for a number of clinical programs.
Both of these types of documents are stored once in a specific area of eTMF and then associated with study-specific Study Items. They are not copied but instead are linked to the Study Items. If study items for a study include enterprise or program documents, the Study Owner is responsible for linking the documents to the appropriate study items.
NOTE: The enterprise/program level is specified for a document type in the master list. Permissions to create program and enterprise documents vary by organization.
Viewing Program and Enterprise Documents
To view Program and Enterprise documents, select the “View Program/Enterprise Documents” option from the More menu in the primary navigation header.
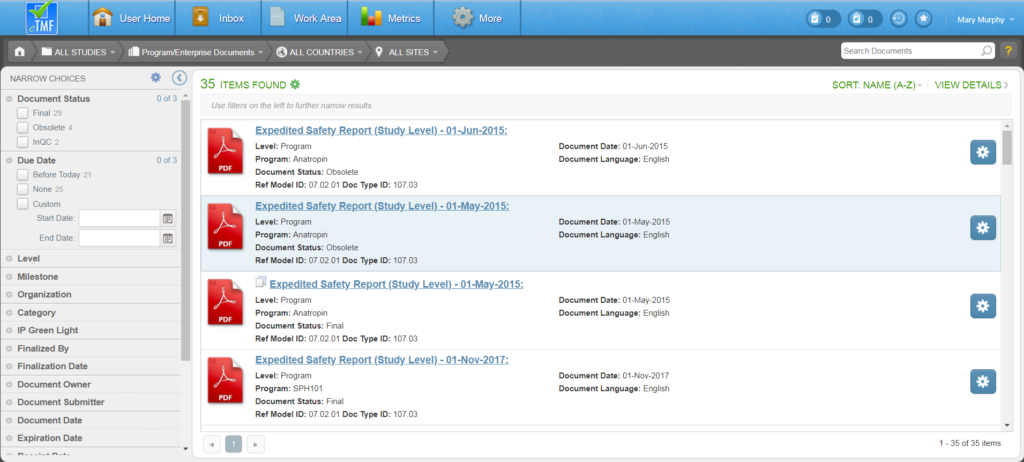
The Program/Enterprise Documents page displays all program and enterprise documents. Study Owners will see documents for the programs to which their studies belong; non-Study Owners will see only documents that are actually associated with their studies.
You can only view Program and Enterprise documents from this page. See Associating Program Level Documents, Associating Enterprise Level Documents, Disassociate Program Document, or Disassociate Enterprise Document for details.
