You can set your out-of-office status in eTMF to automatically delegate your tasks to another user. With the appropriate permissions (i.e, Manage Users feature permissions), you can also set out-of-office status for another user.
To set an office status, navigate to the right side of any page in the eTMF and select your user name to display an associated drop-down menu. From the menu, select “Set Out of Office.”
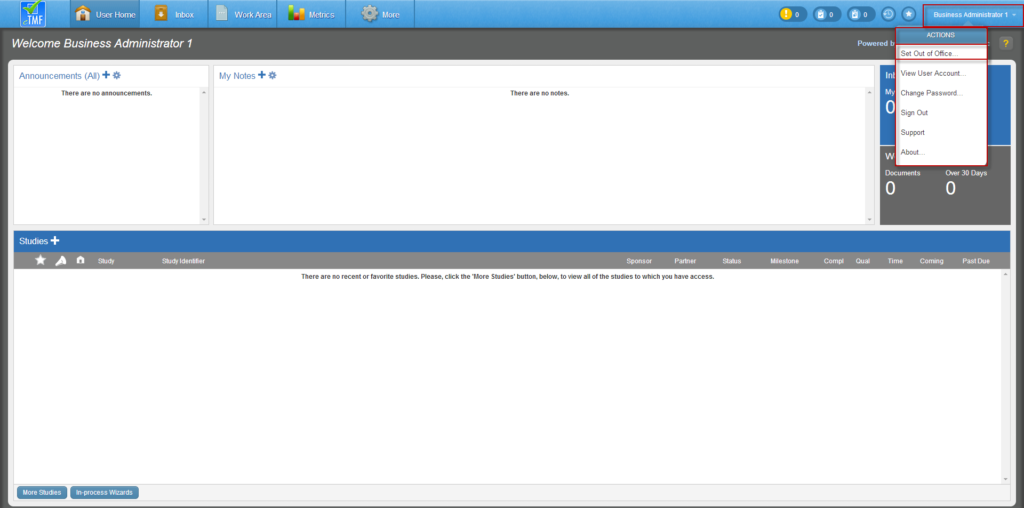
The Set Out-of-Office dialog will display.
Set Out-of-Office Status
To set a user to have the status of ‘out-of-office’, select the “Yes” option for the Is Out-of-Office Enabled toggle in the Set Out-Of-Office window.
To set an out-of-office status for an indefinite period of time, select the “No” option for the Is Using Out Of Office Range toggle. To set a timeframe for the out-of-office status, select “Yes” for the Is Using Out Of Office Range toggle. Then, select a desired start date/time and end date/time using the associated fields.
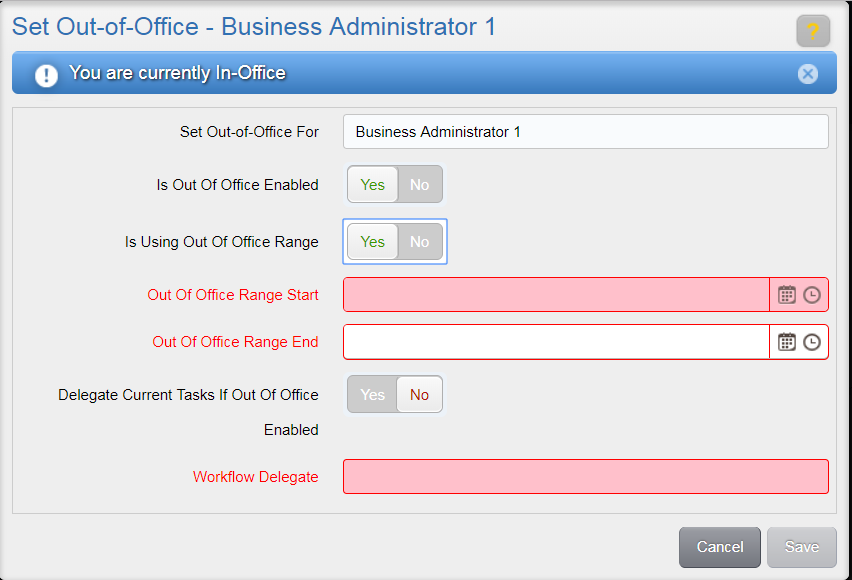
Indicate whether or not you want to delegate tasks that are already in your inbox by selecting either “Yes” or “No” in the toggle associated to the Delegate Current Tasks if Out of Office Enabled option.
Then, select a user to delegate all current (if applicable) and future tasks to while out of office by using the Workflow Delegate drop-down. All tasks sent to you while you are out of office will then be sent to the user you selected. Available users are those with the same role(s) as the out-of-office user. If the out-of-office user has study-specific access, the available users are also limited to those with access to the same studies.
Click Save. The window will close. If you have set yourself as out-of-office, an Out-of-Office icon will be displayed next to your username in the top right corner of the page. If you have set another user as out-of-office, the icon will be displayed in the same location for that user when they log into the system.
Manually End Out-of-Office Status
To manually end your out-of-office status (e.g. if you selected No in the Is Using Out Of Office Range toggle when initiating the Out-of-Office status), navigate to the right side of any page in the eTMF and select your user name to display an associated drop-down menu. From the menu, select “Set Out of Office.”
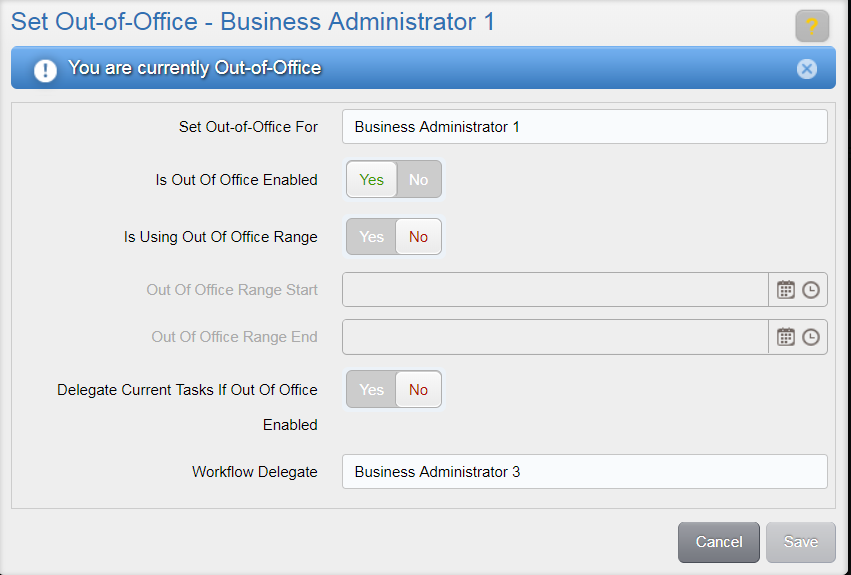
The Set Out-of-Office window will show that the user is currently Out-of-Office.
Select “No” for the Is Out of Office Enabled toggle and click Save.
The Set Out-of-Office window will close, tasks will no longer be delegated to the designated Out-of-Office recipient, and the Out-of-Office icon will no longer display next to the user’s username in the top right corner of the eTMF.