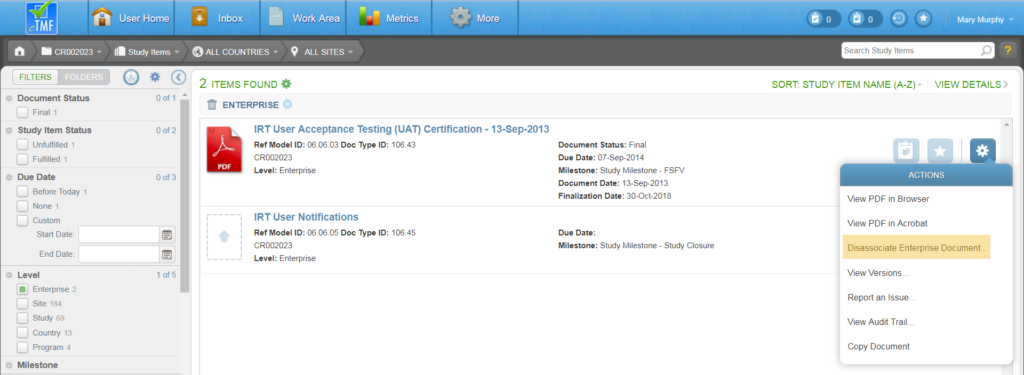
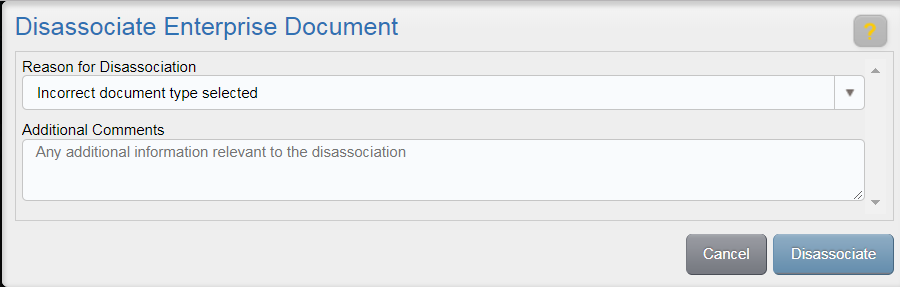
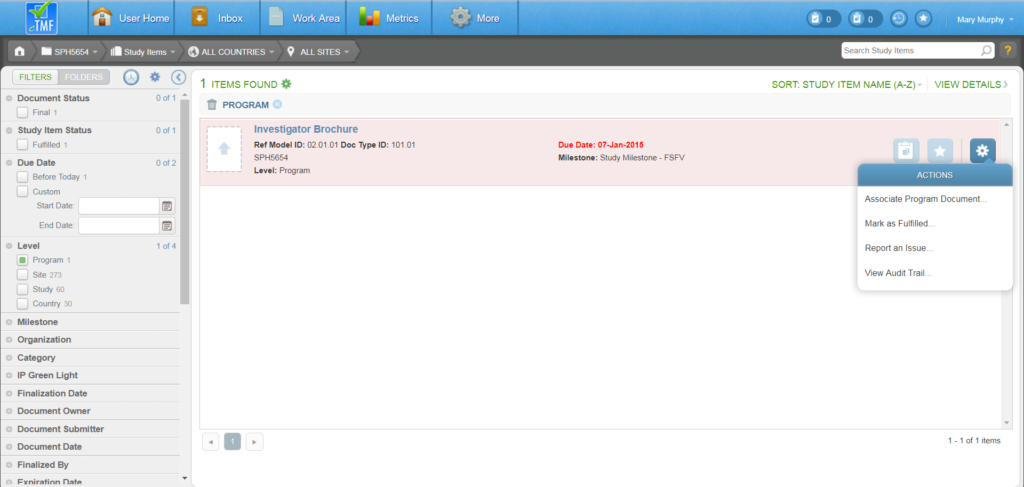
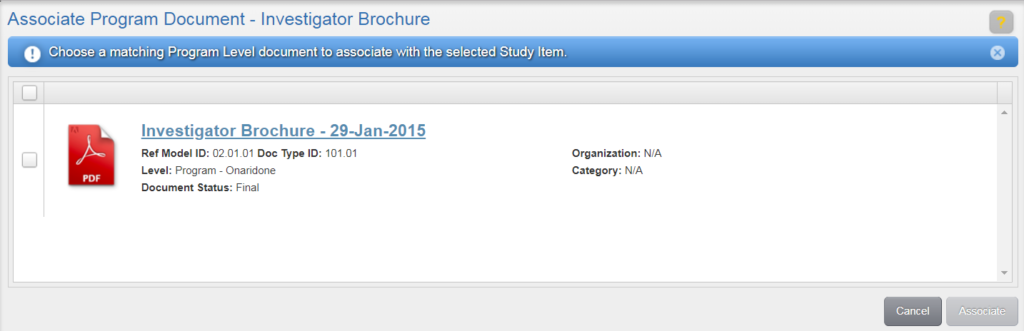
A study can be locked as a way to both prevent the study from modification and prepare it for archiving. Locking a study inhibits a user from making any changes for a study, including: Uploading content, deleting study items, fulfilling study items, obsoleting study items, and running wizards for the study that is locked. Users with Check Lock Preparedness permission can lock a study.
Review the Lock Readiness Report
Before locking a study, review the Lock Readiness report. Generate the report by executing the following steps:
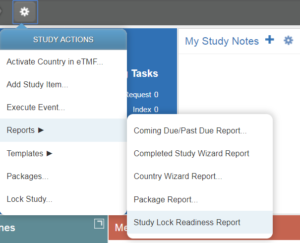
- Navigate to the Study Home page of the study you want to lock.
- Click on the Actions menu icon and select Reports >Study Lock Readiness Report.
The report downloads to either your default download location or to a location you specify. The exact behavior is based on the browser used.
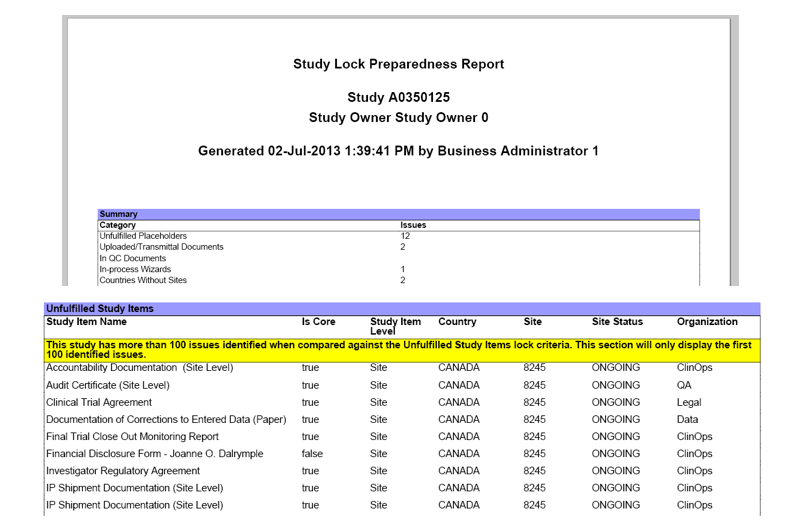
The report contains a summary page that displays counts of issues in the following categories:
- Unfulfilled Study Items
- Documents in Work Area
- Documents in Workflow
- In-process Wizards
- Countries with No Sites
- Sites not in TMF
- Unaddressed Acknowledgements
The report also shows the breakdown of issues within each category.
Complete all required and desired remediation
If there are issues outlined in the Study Lock Readiness Report, complete all required and recommended remediation based on the following guidance:
| Issue | Action | Must be Addressed? |
|---|---|---|
| Unfulfilled Study Items | Determine why they are empty and perform one of the following options: * Obtain Content * Mark as Fulfilled without Content * Delete | No |
| Uploaded/Transmittal Documents | Determine if content is needed. * If not needed, delete using Advanced Search * If needed, contact contributors to release the documents to QC | Yes |
| In QC Documents | Contact task owners to expedite the completion of tasks. | Yes |
| In-Process Wizards | Determine if the wizard needs to be finished and if the resulting content is needed in the eTMF * If needed, complete the wizard and address missing documents * If not, delete the wizard | Yes |
| Countries Without Sites | Determine if content is needed for sites * If it is not needed, no action is necessary * If it is necessary, complete the site wizard(s) and address the missing documents | No |
| CTMS Sites Not in the TMF | Determine if content is needed for the sites * If not needed, no action is necessary * If it is needed, complete the site wizard(s) and address missing documents | No |
All essential issues must be addressed in order to lock the study.
Lock Study
To lock the study, navigate to the Study Home page of the study you want to lock. Click on the Actions menu icon and select “Lock Study.”
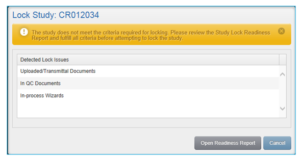
If the required criteria for locking has not been met, a warning message will display, stating that you must review the Lock Readiness Report and fulfill all criteria before you can lock the study. Click Open Readiness Report to view the report immediately or Cancel to generate the report at a later time.
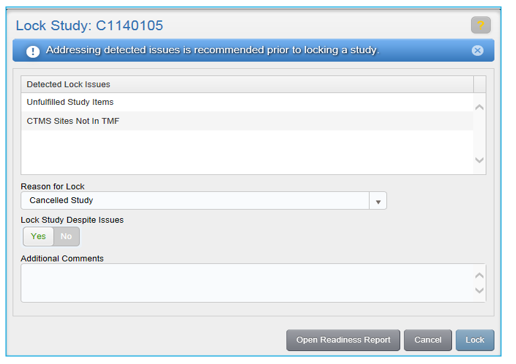
If only non-essential issues remain, you can proceed with locking the study by acknowledging that you are aware of the issues and providing a reason for the lock. Select a reason from the Reason for Lock drop-down, and select “Yes” in the Lock Study Despite Issues field. Enter any Additional Comments you might have and click Lock.
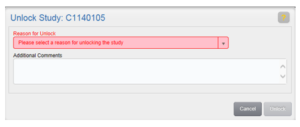
Unlock a Study
Once a study has been locked, it can be unlocked if you need to add additional study items or fulfill study items. To unlock a study, you must have Study Unlock permission.
- Navigate to the Study Home page of the study you want to lock.
- Click on the Actions menu icon and select “Unlock Study.”
- Select a Reason for Unlock, enter Additional Comments if necessary, and click Unlock.