Packages can be created within a study to track a subset of documents in the TMF for a specific purpose (e.g., drug shipment, Health Authority submission).
Packages are created within the Package List Page of a study. You can access the Package List Page by navigating to the Study Item List and then selecting “Package List” from the Study Items Breadcrumb Menu.
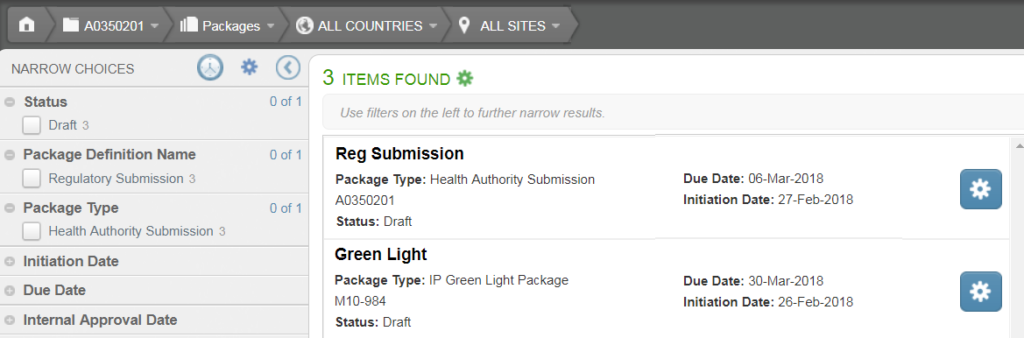
If you are a member of the TMF View Packages security group, the Package List Page displays all approved and rejected packages. Members of the TMF Create Packages or TMF Manage Package Definitions security groups can additionally see all draft packages.
Under the package name, the type, status, due date, and initiation date are displayed. If the package has an approval or rejection status, you’ll see additional information related to the approval or rejection.
From the Packages List Page, you can view the package specifications, view package versions, or export its contents or metadata. If you are a member of a Creator Group* for the package, you can additionally create new packages or edit draft packages to add or remove Study Items. If you are a member of an Approval Group* for the package, you can additionally approve packages once all the Study Items have been fulfilled and finalized. Approval can be internal or external if provided from an outside party.
* Note: Creators and Approvers are specified within the Package Definition on which the package is based. These users also need to be members the TMF Create Packages security group to have access to draft packages.
